

Adv. Mater.2024, 2400523
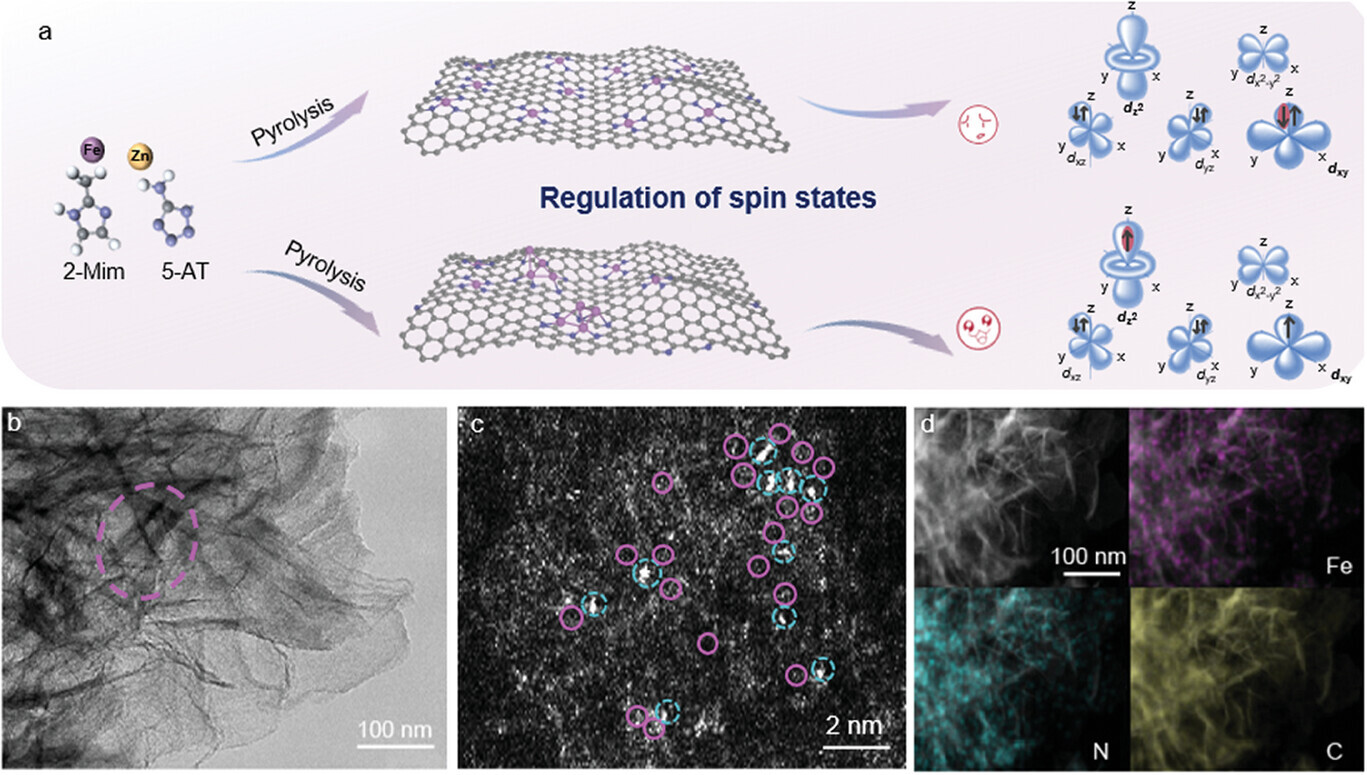
The interaction between oxygen species and metal sites of various orbitals exhibits intimate correlation with the oxygen reduction reaction (ORR) kinetics. Herein, a new approach for boosting the inherent ORR activity of atomically dispersed Fe−N−C matrix is represented by implanting Fe atomic clusters nearby. The as-prepared catalyst delivers excellent ORR activity with half-wave potentials of 0.78 and 0.90 V in acidic and alkaline solutions, respectively. The decent ORR activity can also be validated from the high-performance rechargeable Zn–air battery. The experiments and density functional theory calculations reveal that the electron spin-state of monodispersed Fe active sites is transferred from the low spin (LS, t2g6 eg0) to the medium spin (MS, t2g5 eg1) due to the involvement of Fe atomic clusters, leading to the spin electron filling in σ∗ orbit, by which it favors OH− desorption and in turn boosts the reaction kinetics of the rate-determining step. This work paves a solid way for rational design of high-performance Fe-based single atom catalysts through spin manipulation.