

Adv. Sci.2024, 2309526
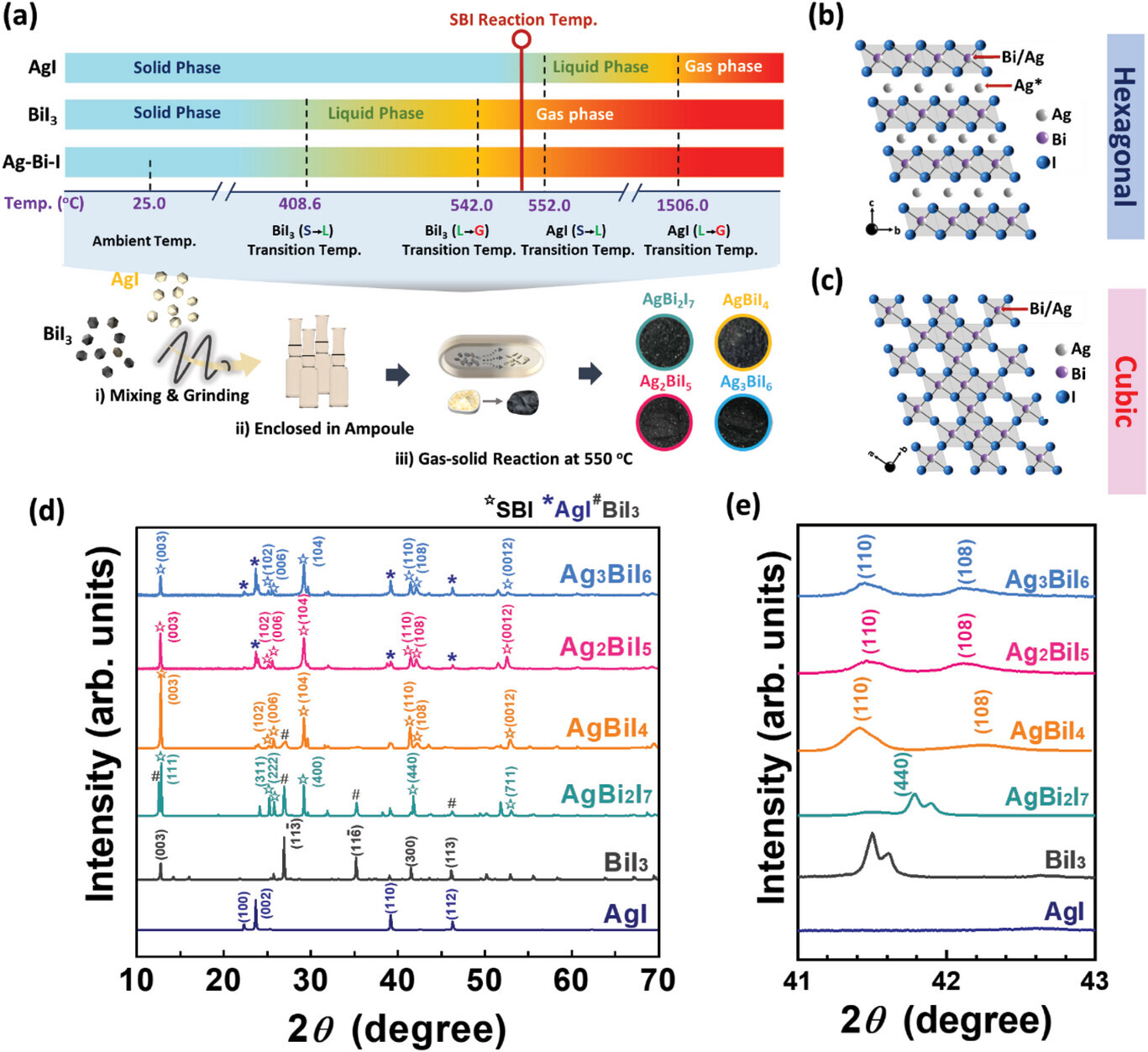
Photocatalytic reduction of CO2 is a promising strategy to mitigate the effects of global warming by converting CO2 into valuable energy-dense products. Silver bismuth iodide (SBI) is an attractive material owing to its tunable bandgap and favorable band-edge positions for efficient CO2 photoreduction. In this study, SBI materials, including AgBi2I7, AgBiI4, Ag2BiI5, and Ag3BiI6 are first synthesized, through gas-solid reaction by controlling the stoichiometric ratio of reactants. The X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) results revealed that the distance between Ag-I is proportional to the degree of Ag ions delocalization, which occupies the vacant sites. That greatly retards the charge recombination at vacant sites. In addition, the surface potential via photo-assisted Kelvin probe force measurements of various SBI catalysts shows that Ag3BiI6 exhibits the highest surface potential change due to the rich delocalized Ag ions. This results in effective charge carrier transport and prevention of charge recombination at vacant sites. Taking the above advantages, the averaged CO and CH4 production rates for Ag3BiI6 achieved 0.23 and 0.10 µmol g−1 h−1, respectively. The findings suggest that Ag3BiI6 has a high potential as a novel photocatalyst for CO2 reduction and sheds light on the possibility of solving environmental contamination and sustainable energy crises.