

J. Colloid Interface Sci. 586 (2021), 514-527
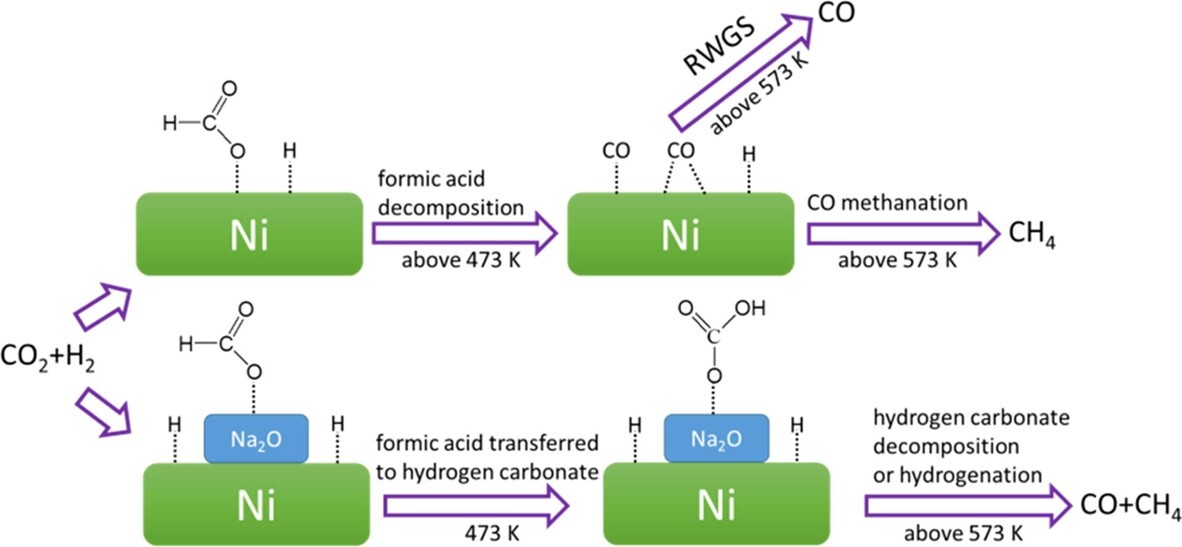
CO2 hydrogenation over Ni/SiO2 catalysts with and without Na additives was investigated in terms of the catalytic activity, selectivity of CO2 methanation and reaction mechanism. Na additives could cause the formation of Na2O species that might deposit on the Ni surface of Ni/SiO2 (NiNax/SiO2). When the Ni metal is partially covered with Na2O species, a highly positive charge on the Ni metal could occur compared to the original Ni/SiO2 catalyst. The addition of Na to the Ni/SiO2 catalyst could influence selectivity toward CO formation. The adsorbed formic acid is the major intermediate on the Ni/SiO2 catalyst during CO2 hydrogenation. The formic acid species might decompose into adsorbed CO complexes in the forms of linear CO, bridged CO and multibonded CO. CH4 formation should be ascribed to the hydrogenation of these adsorbed CO complexes. The Ni/SiO2 catalyst with the Na additive might have very weak ability for H2 and CO adsorption, thus making it difficult for CO methanation to occur. The hydrogen carbonate species adsorbed on the NiNax/SiO2 catalysts were proposed to be the key intermediate, and they might decompose to CO or be hydrogenated to form CH4.
https://doi.org/10.1016/j.jcis.2020.10.117