

Effect of sodium promoters on Ni/Al2O3 catalyst for CO2 hydrogenation: The carbon fixation as carbon nanofiber and reverse-water gas reactions
Chem. Eng. J. 478 (2023), 147373
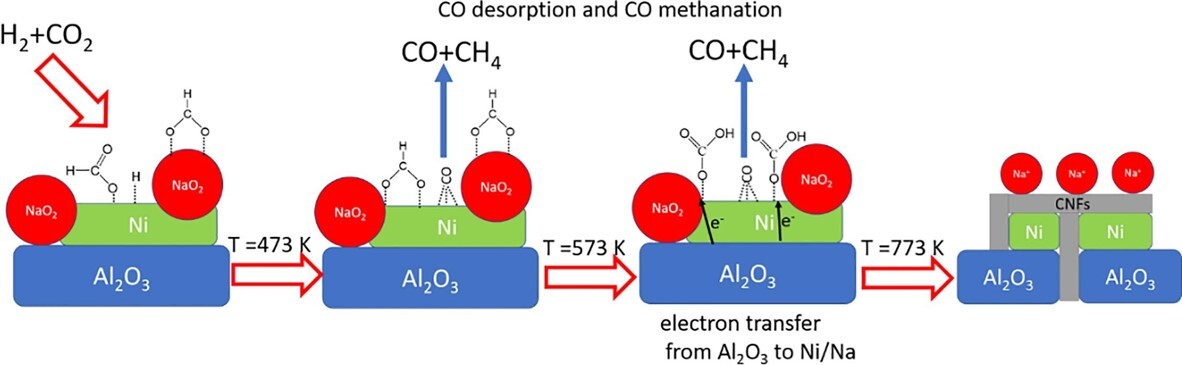
Reducing CO2 emissions has become a critical priority in addressing the environmental impact of carbon dioxide. Technologies for CO2 fixation primarily involve carbon capture storage and utilization (CCSU) and chemical conversion, typically pursued along independent tracks. In our research, we have focused on developing a catalytic reaction for CO2 hydrogenation that integrates the benefits of both CCSU and chemical conversion. The use of sodium-promoted Ni/Al2O3 catalyst (NiNax/Al2O3) in the CO2 hydrogenation process exhibits a synergistic effect, leading to the simultaneous production of CO, CH4, and carbon nanofibers (CNFs). Notably, the conversion of CO2 into CNFs represents a novel approach for carbon fixation, in addition to the formation of CO and CH4. Importantly, the formation of CNFs on the NiNax/Al2O3 catalyst does not compromise its catalytic activity for CO2 hydrogenation to CO and CH4, which is a noteworthy finding. This bifunctional catalyst has the potential to offer significant advantages, including high CO production and the generation of valuable carbon materials. The CNFs are predominantly formed at the interfacial sites between NiNax and Al2O3. The formation process involves the reaction between H2 bound to Ni and adsorbed CO2, leading to the formation of hydrogen carbonate species, which subsequently give rise to CNFs. The reduction of CO2 to CNFs is facilitated by the transfer of electrons from Al3+ species to the interfacial sites between NiNax and Al2O3.